american academy of pediatrics covid vaccine 5-11
This is a difficult question without an easy answer something you wouldnt know from the strident opinions of politicians and health experts. Almost one-fourth of all kids 5 through 11 have had at least one dose.

Covid 19 Vaccines For Children 5 To 11 Uchicago Medical Experts Address Common Questions University Of Chicago News
Is the Pfizer BioNTech mRNA vaccine.
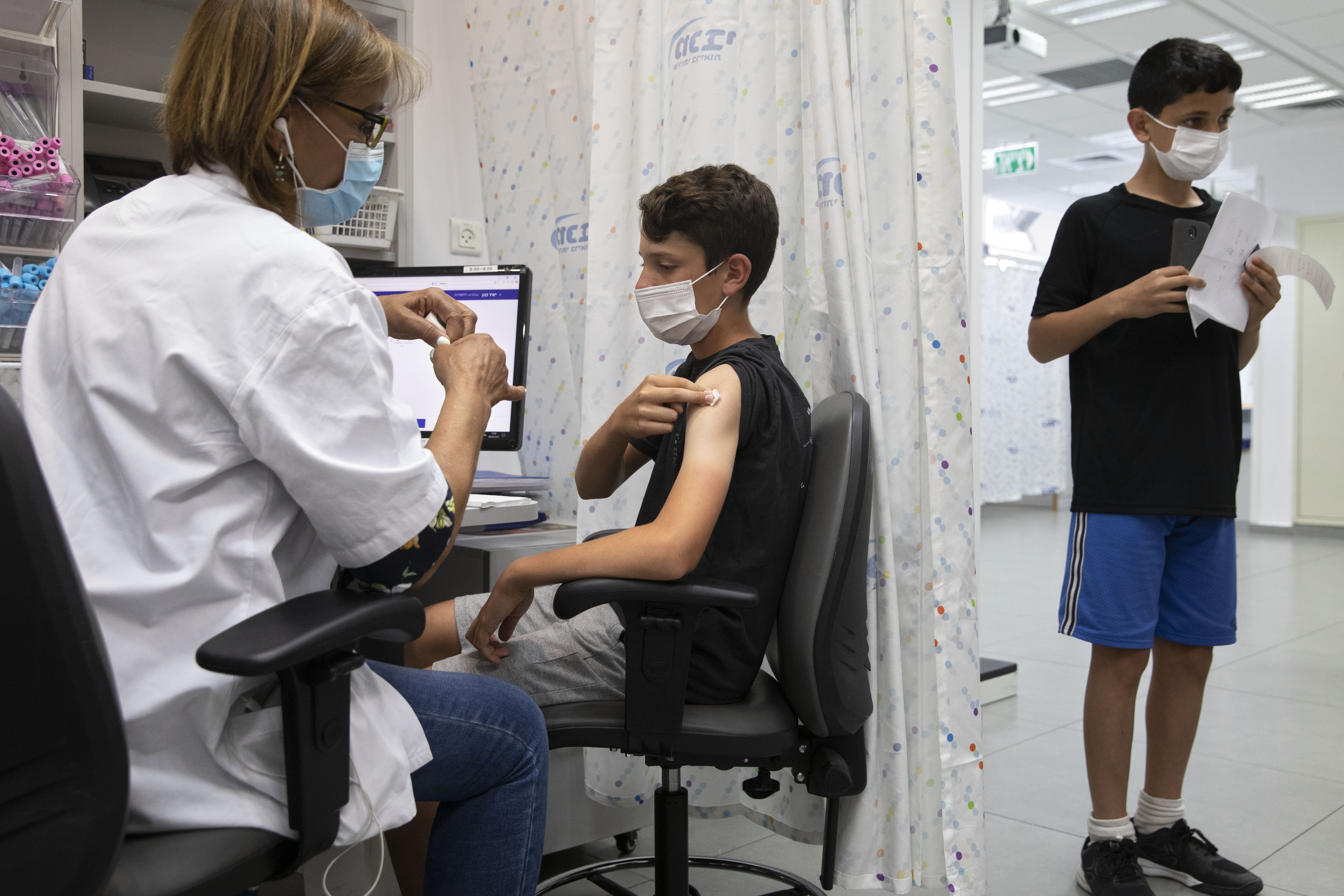
. Recently the Centers for Disease Control and Prevention CDC gave emergency use authorization of the Pfizer COVID-19 vaccine for children 5-11 years old. The vaccines cannot give you COVID. Vaccine trial data on efficacy safety.
The vaccines continue to be monitored very closely. The most common side effect was a sore arm. Currently children ages 5 through 11 years are eligible to receive the Pfizer-BioNTech COVID-19 vaccine.
About 28 of children ages 5-11 years and 58 of those ages 12-17 years are fully vaccinated according to the CDC. 2 days agoShareTweetSharePin0 Shares Should children ages 5 to 11 receive the COVID-19 vaccine. The COVID shot for children 5 years to 11 years old is a lower dose than the dose recommended for people 12 years and older.
They can reduce the risk of severe illness from COVID. According to the president of the. Heres a checklist as you prepare for your childs COVID-19 vaccination.
At this point Pfizers under-5 vaccine will almost certainly be a three-doser at least to start and the company is evaluating a third dose in 5. Federal health officials gave final approval to using the Pfizer-BioNTech vaccine for about 28 million children in this age group Tuesday and the AAP released a policy statement recommending all eligible children without contraindications get vaccinated. 2 2021 Pediatricians can start vaccinating children ages 5-11 years against COVID-19.
More than 60 of kids ages 12-17 and at least 25 of kids ages 5-11 in the US. COVID-19 vaccine for children ages 5-11 receives final approval Nov. A survey last week from the Kaiser Family Foundation found 66 of parents of 5- to 11-year-olds were worried the COVID-19 vaccine would cause infertility issues in their children when theyre older.
Information from the CDC on clinical considerations for COVID-19 vaccines. Have had at least one COVID-19 vaccine. It was the eighth leading cause of death for children in this younger age group.
American Academy of Pediatrics urges FDA to approve vaccine for kids 5-11 Dr. Lee Savio Beers from the American Academy of Pediatrics talks about the rise in. Updated 152022 COVID-19 Vaccine for Children Ages 5-11 The American Academy of Pediatrics AAP Advisory Committee on Immunization Practices ACIP Centers for Disease Control and Prevention CDC and the North Dakota Department of Health NDDoH all recommend COVID-19 vaccination of children and adolescents 5 years of age and older.
Thats over 13 million kids who have had both of their doses of COVID-19 vaccine. In clinical trials vaccine side effects were mild self-limiting and similar to those seen in adults and with other vaccines recommended for children. A 5-year-old girl receives her first dose of the Pfizer COVID-19 vaccine from a nurse in the cafeteria of the Pittsburgh Langley K-8 school in Pittsburgh on Jan.
A booster dose is also strongly encouraged for ages 12 and up who got two doses of the mRNA COVID vaccine at least five months ago. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. Once eligible children ages 6 months to 4 years will receive a separate vaccine from children.
With children ages 5 to 11 soon to be eligible for the vaccine Lee Savio Beers the academys president this year spoke with USA TODAY about the organizations work how to stay safe in school. There have been more than 19 million COVID-19 cases reported in children 511 with more than 8300 hospitalizations and almost 100 deaths since the pandemics onset. COVID Vaccine Safe for Kids Whove Had MIS-C Small Study Suggests - Consumer Health News HealthDay Posted by Georgia Chapter American Academy of Pediatrics at 3302022 090500 AM.
Have been fully vaccinated. The dosage is one-third of the adolescent and adult dose. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age.
The COVID-19 vaccine is available to kids 5 years old and up. Ad Safety is CDCs top priority and vaccination is the safest way to help build protection. AAP guidance on providing COVID-19 vaccines to children and adolescents.
5 2021 - The CDC has issued interim clinical considerations to help providers who administer the COVID vaccine to children ages 5-11 years. Children ages 6 months through 4 years may soon become eligible for the Pfizer-BioNTech vaccine. Pediatricians can start vaccinating children ages 5-11 years against COVID-19.
On one side are the Centers for Disease Control and Prevention the American Academy of Pediatrics and Pfizer which makes the. The American Academy of Pediatrics AAP recommends the COVID-19 vaccine for ages 5 and older. The Food and Drug Administration must work aggressively toward authorizing a Covid-19 vaccine for children under age 12 the head of the American Academy of Pediatrics wrote in a letter to Dr.
Over half of all kids 12 to 17 years old in the US. The AAP released a statement about infertility and the COVID-19 vaccine after parents raised concerns about the misinformation. Similar to what was seen in adult vaccine trials vaccination was nearly 91 percent effective in preventing COVID-19 among children aged 5-11 years.
In addition to these physical effects COVID-19 affects childrens quality of life. If approved the pediatric vaccine would be given in two 10-microgram doses administered 21 days apart. CDC guidance for clinicians on myocarditis after COVID-19 vaccination.
COVID-19 Vaccination Recommendations for Pediatric Populations. Clinical trials in children ages 5-11 years found the vaccine to be 907 effective in preventing symptomatic COVID-19. The only COVID-19 vaccine presently available for children older than 5 years in the US.

States Can Reserve Covid 19 Shots For Younger Kids Next Week

Babies Children Vaccinate Your Family

Pediatricians Besieged By Parents Seeking Coronavirus Shots For Kids Under 12 The Washington Post
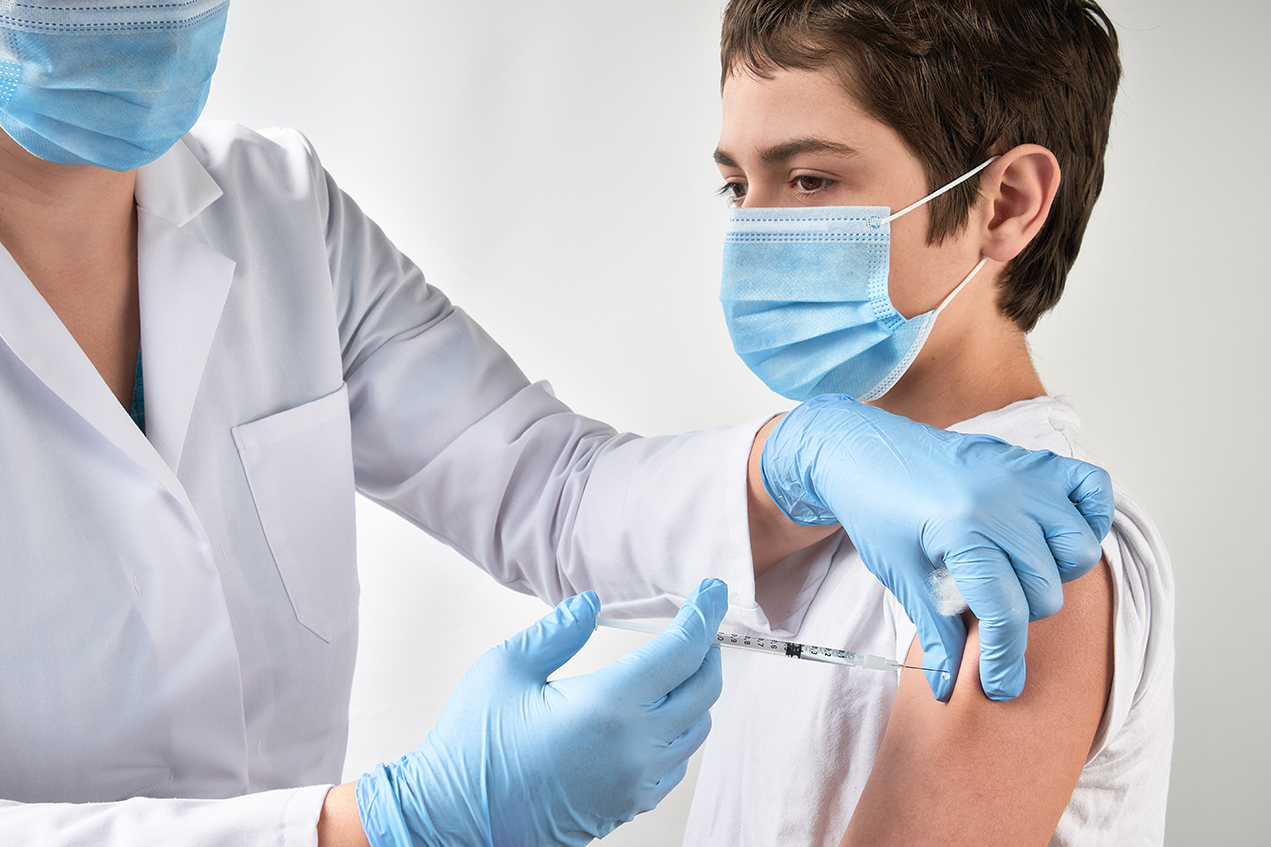
What Parents Need To Know About Covid 19 Vaccines And Kids Hsc News

Pfizer Says Its Covid 19 Vaccine Works For Kids Ages 5 To 11 Will Request Authorization

Everything You Need To Know About The Covid 19 Vaccine For Kids 5 11 In Colorado Colorado Newsline

Pfizer Vaccine Much Less Potent In Kids Aged 5 11 Consumer Health News Healthday
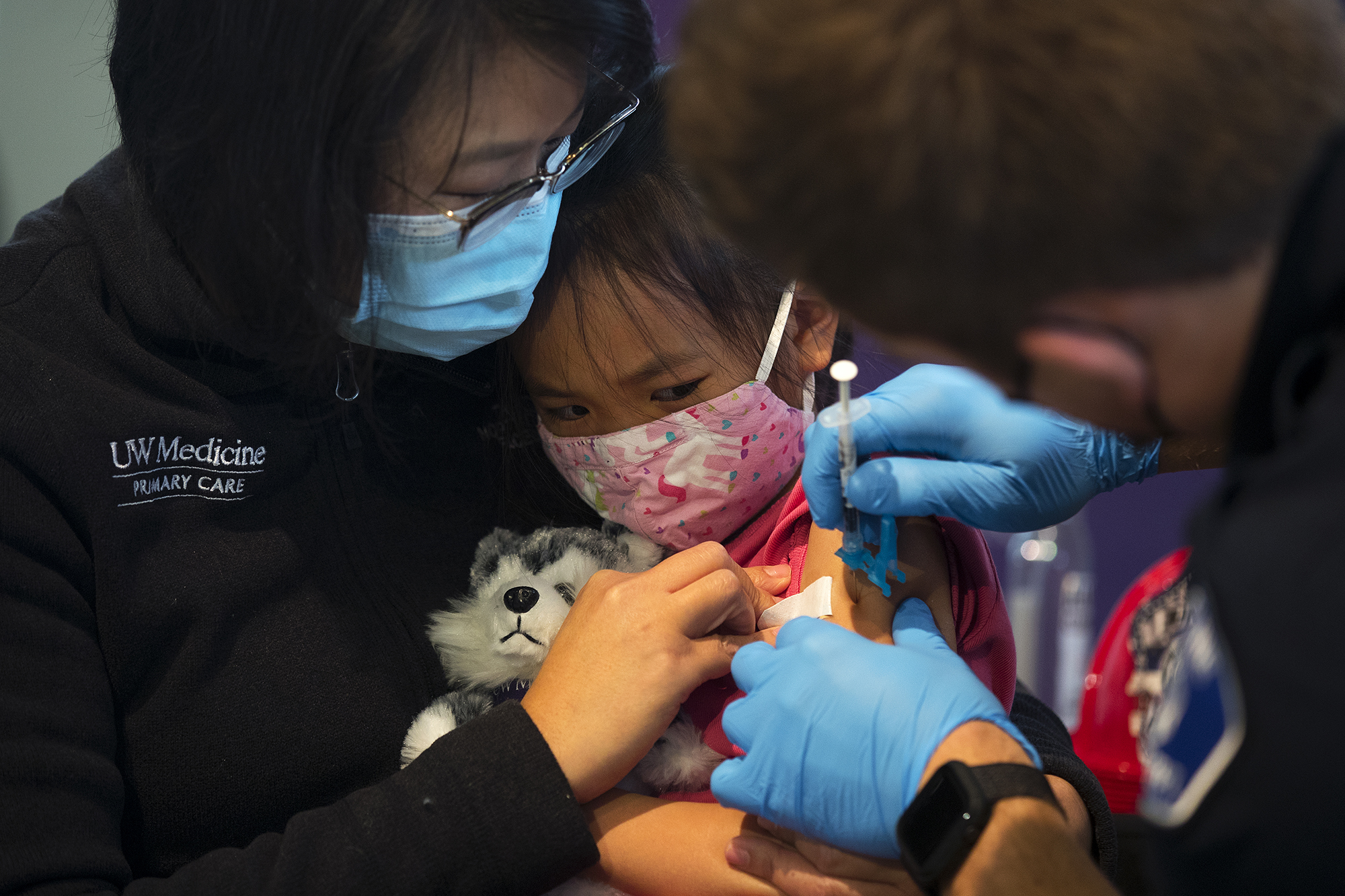
Kuow Want To Get Your 5 11 Year Old Vaccinated Against Covid Here S What You Need To Know
Us Children Aged 5 To 11 On Track To Receive Pfizer Vaccine By Halloween Vaccines And Immunisation The Guardian

What To Know With Covid Vaccines For Kids Under 12 Now One Step Closer Nbc Chicago
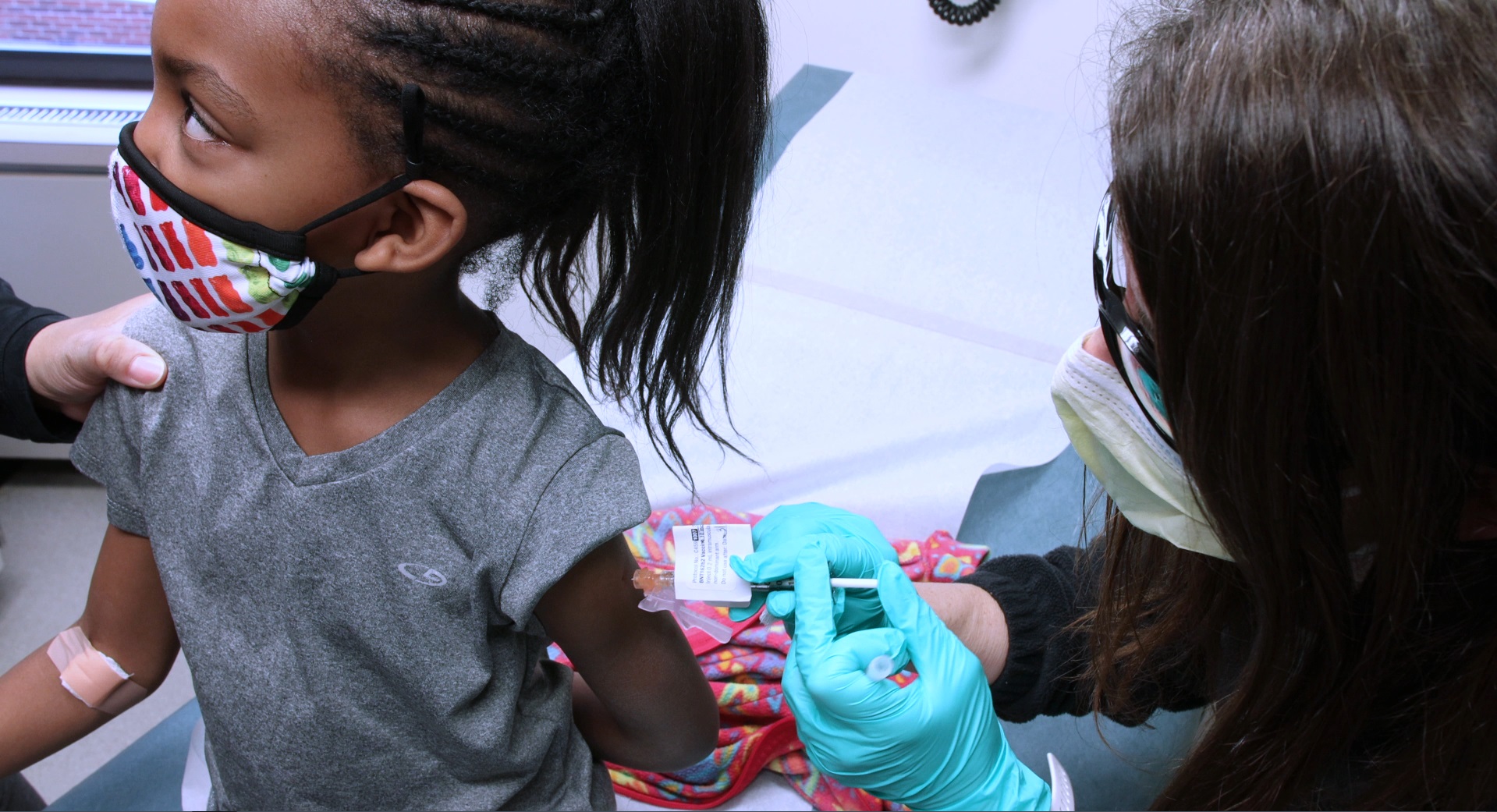
Moderna Pfizer Must Expand Clinical Trials Of Covid Vaccines To Children Ages 5 To 11 At Request Of Fda Masslive Com

Real World Data Confirms Pfizer Vaccine Safe For Kids Ages 5 11 Consumer Health News Healthday
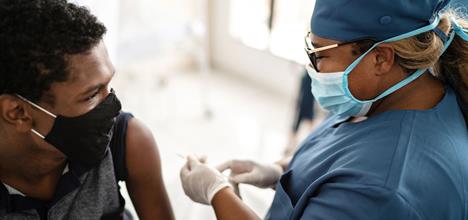
How Long Do Kids Teens Need To Wait Between Each Covid Shot Healthychildren Org

Fcps To Hold More Vaccination Clinics At Several Locations

Cdphe Recommends Parents And Guardians Plan For Covid 19 Vaccines For 5 11 Year Olds Colorado Covid 19 Updates

Covid 19 Vaccine Uptake Toolkit Alabama Chapter Of The American Academy Of Pediatrics

What Do We Know About Covid 19 In Kids Now Witf
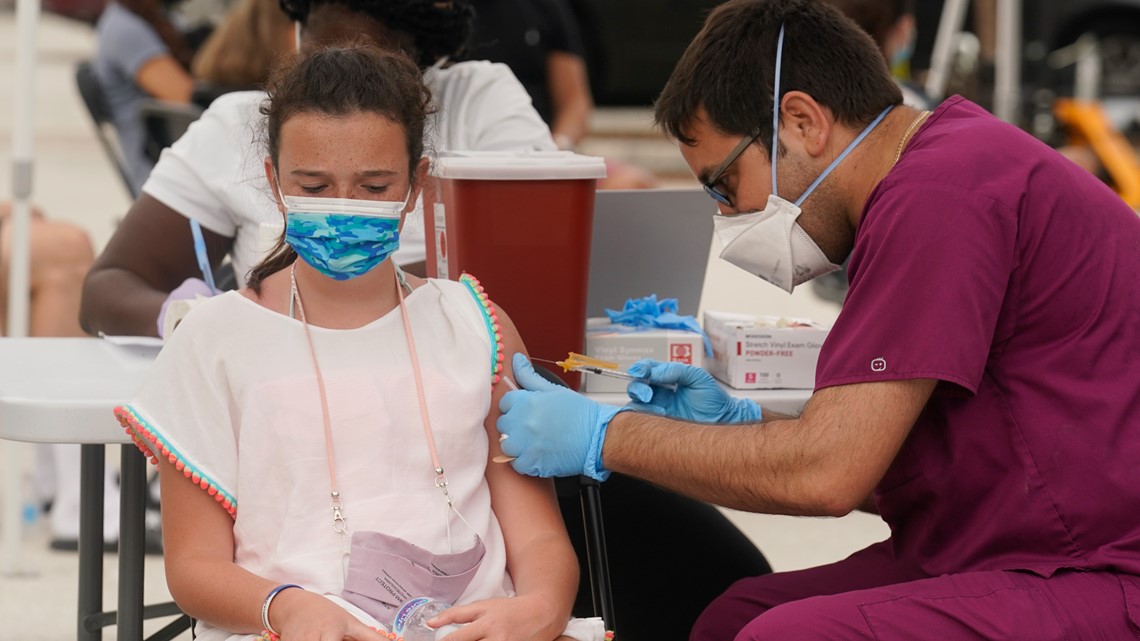
When Will Covid Vaccines Be Available For Kids Under 12 11alive Com
